TABLET CRYSTALLINITY ANALYSIS
About the sample: Amorphous API tablets
Amorphous API (Active Pharmaceutical Ingredients) can increase the dissolution rate and enhance drug release. It improves the bioavailability and bioactivity of solid APIs and offers new opportunities to design more effective and stable medications. However, many APIs are prone to crystallize, and it can be challenging to keep them in an amorphous state. X-ray CT (computed tomography) can visualize crystallization of such APIs non-destructively to understand how crystallization occurs and quantify the crystalline phase.
Analysis procedure
- In this example, amorphous fenofibrate tablets spiked with crystalline fenofibrate were scanned using a submicron-resolution CT scanner, nano3DX.
- The crystalline phase was segmented in the CT images.
- The crystallinity was calculated from the segmentation analysis results.
1. CT scan
Three amorphous fenofibrate tablets were scanned to produce the 3D grayscale CT image.
The difference of density between the matrix (amorphous fenofibrate and polymer mixture) and the crystalline fenofibrate phase was estimated to be 8%. The obtained CT images are colored in the figure, the matrix blue-green and the crystalline phase yellow.
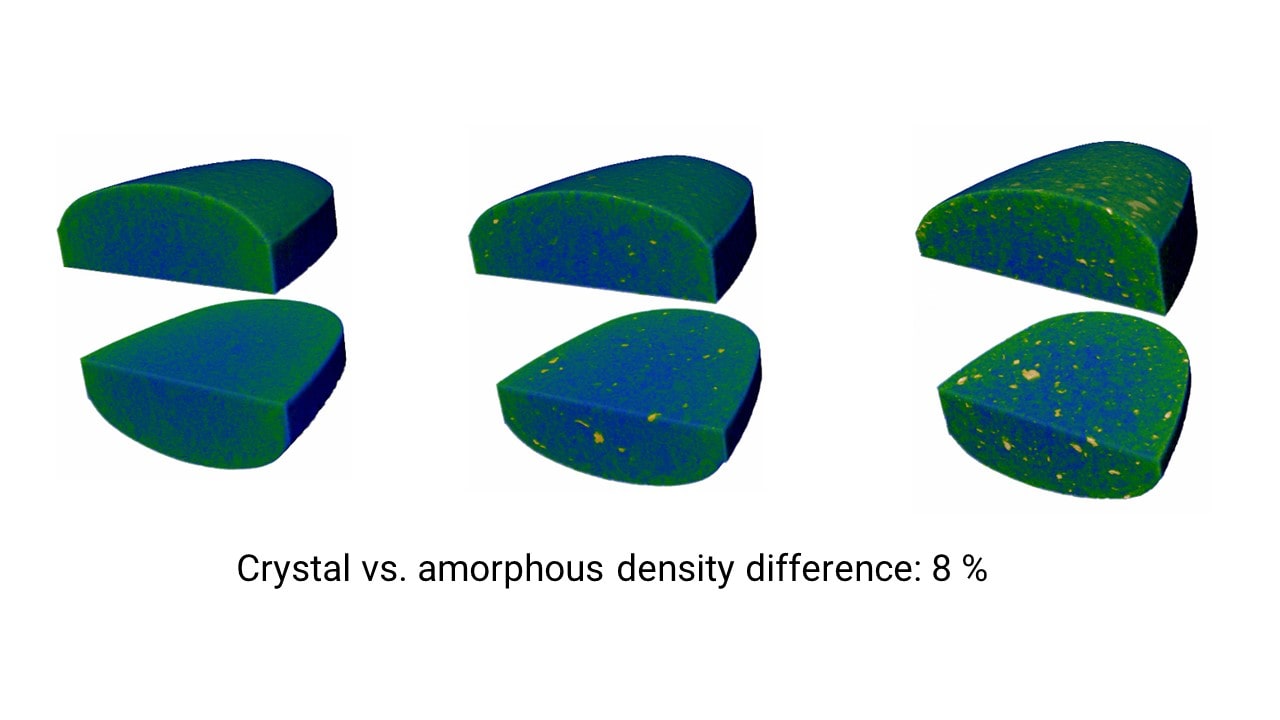
2. Crystalline phase segmentation
The crystalline phase was segmented in the CT images. The segmented phase is shown in red in the figure.
3. Crystallinity calculation
The volume fraction of the crystalline phase was calculated from the image segmentation and converted into weight percent using the material densities. The spiked crystalline amounts were 0, 1, and 3wt%, and they were calculated as 0.08, 0.70, and 2.87wt%, showing good agreement.

This example demonstrates X-ray CT's capability to visualize and quantify crystalline API in an amorphous matrix. For a more thorough evaluation of this technique, see the work by Neilly et al. (2020), J. Pharm. Sci., 109(1), 3078-3085.
More Pharmaceutical Application Examples
Watch an on-demand webinar about X-ray CT pharmaceutical applications.

Tablet crystallinity analysis
Application Note
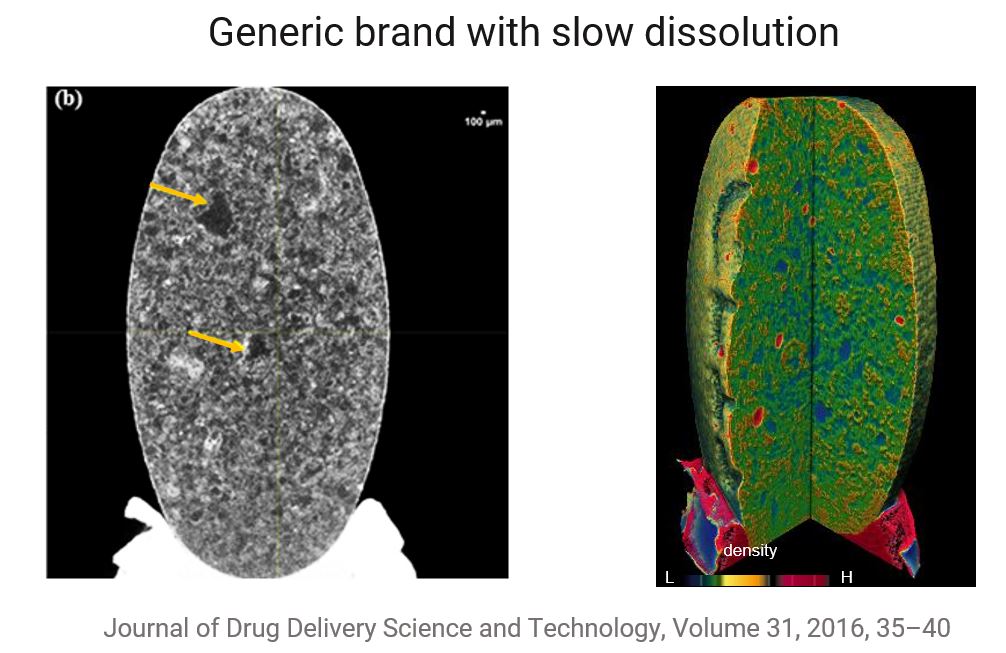
Brand name vs generic atorvastatin tablets comparison
Application Note
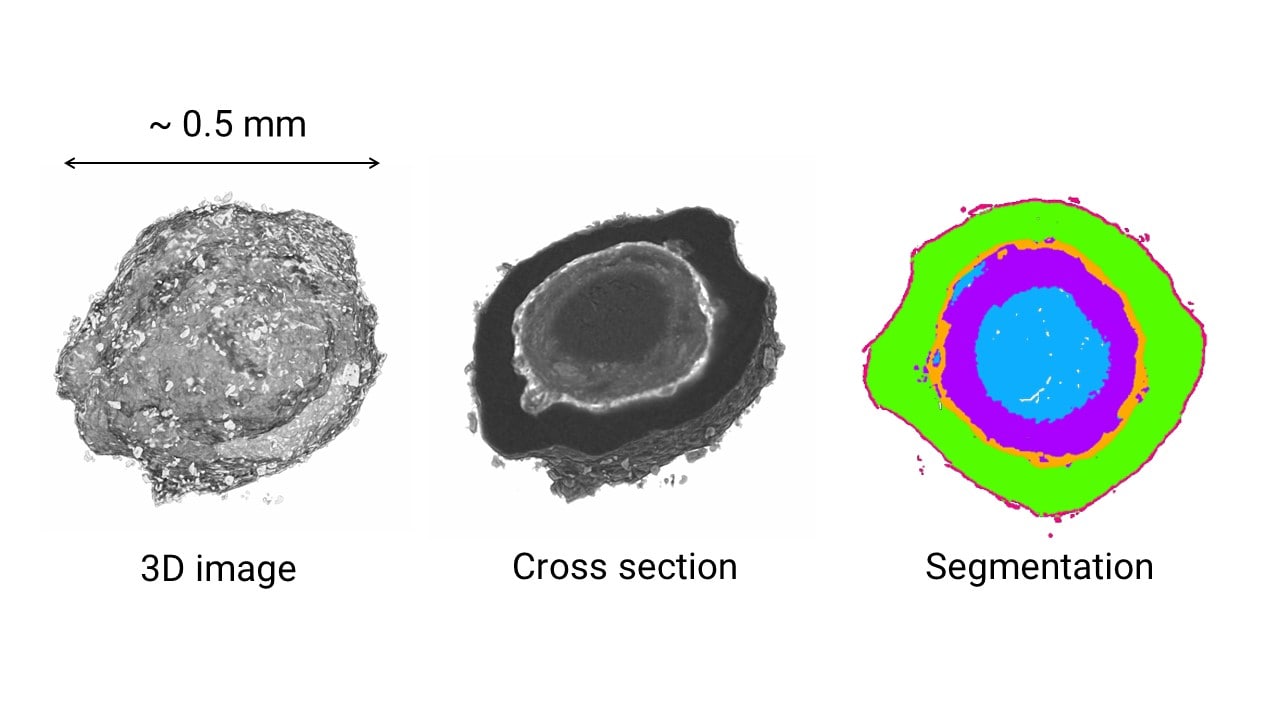
Microparticle coating analysis
Application Note

Rapid-release pain medication capsule comparison
Application Note
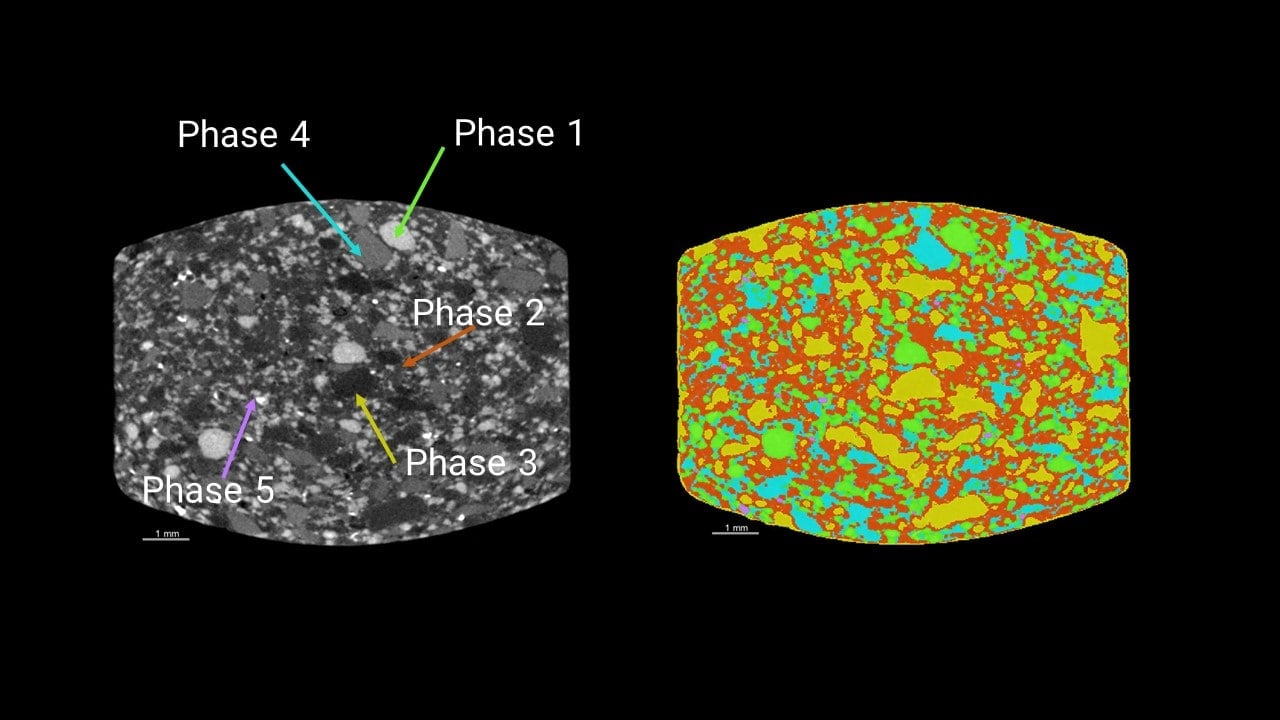
Multivitamin tablet analysis
Application Note
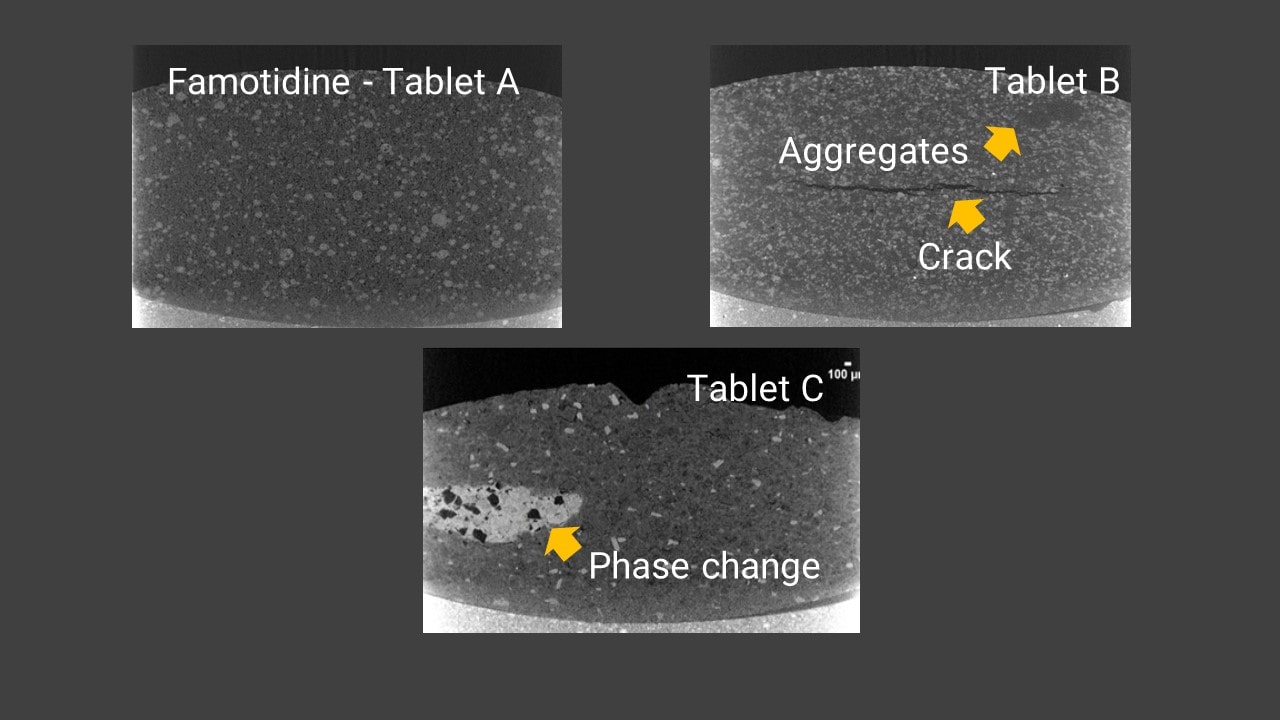
Famotidine tablet comparison
Application Note
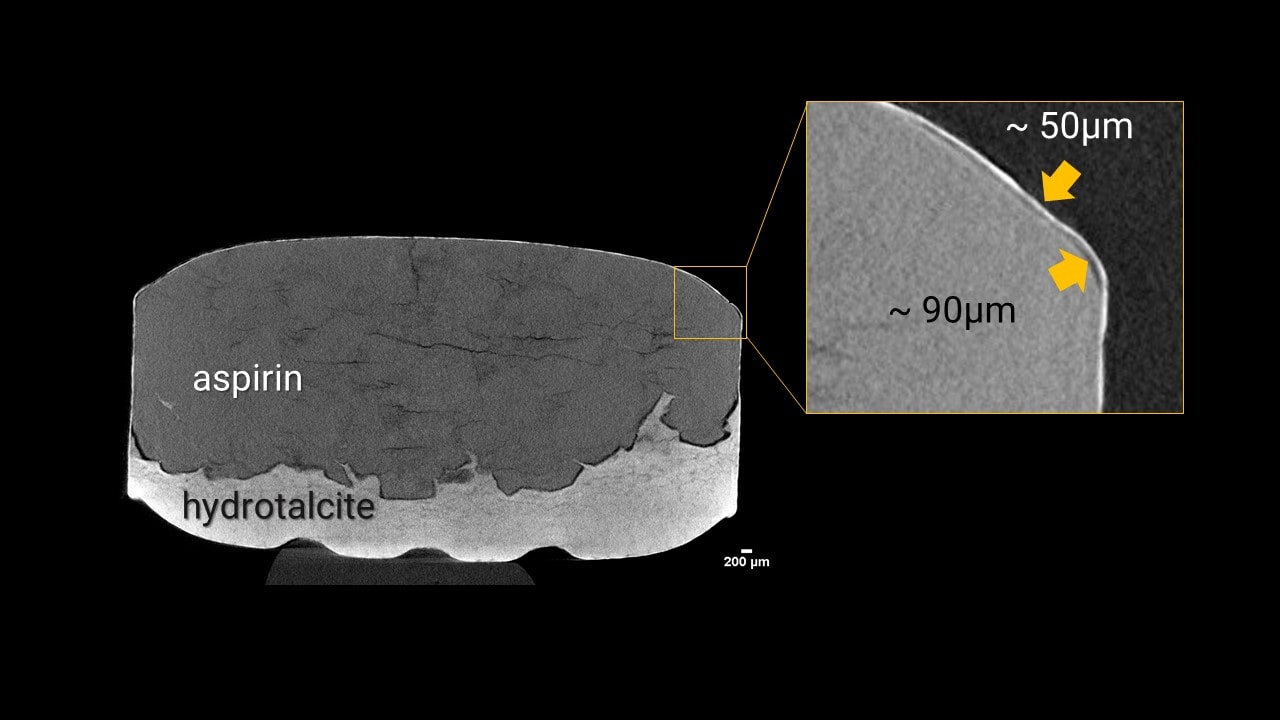
Aspirin tablet coating delamination
Application Note
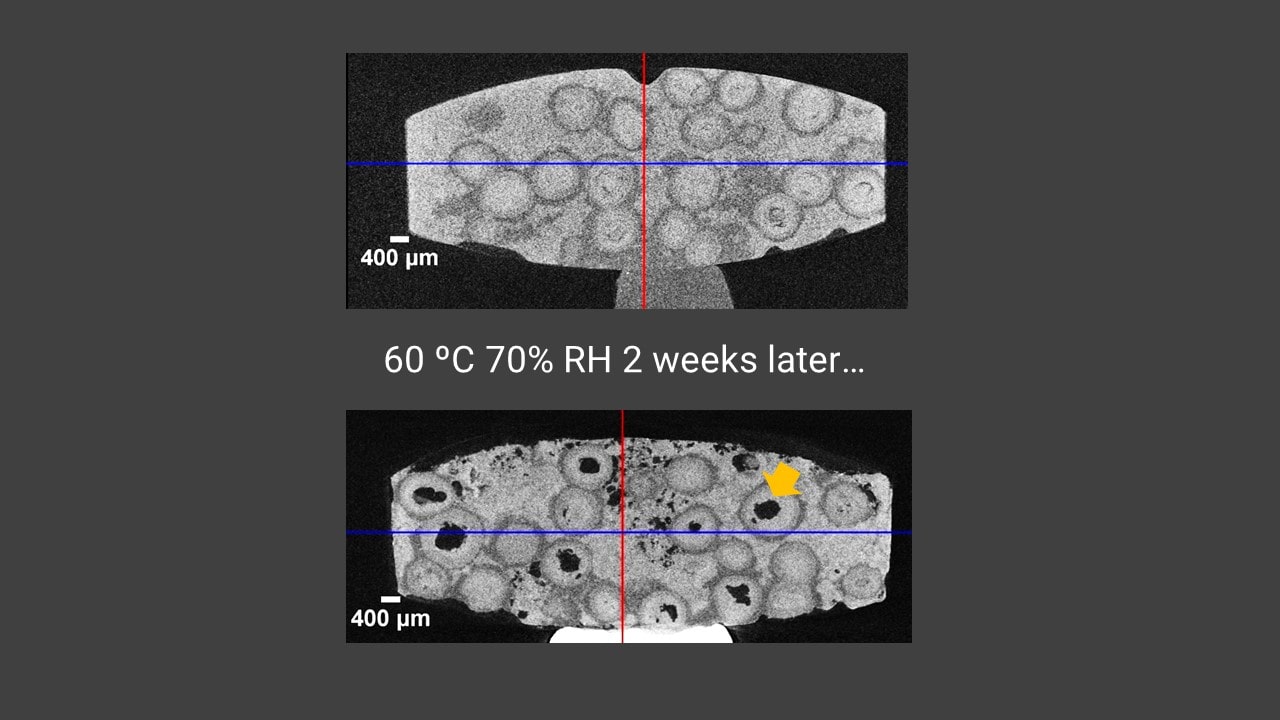
Degradation of sustained-release dosage tablet
Application Note
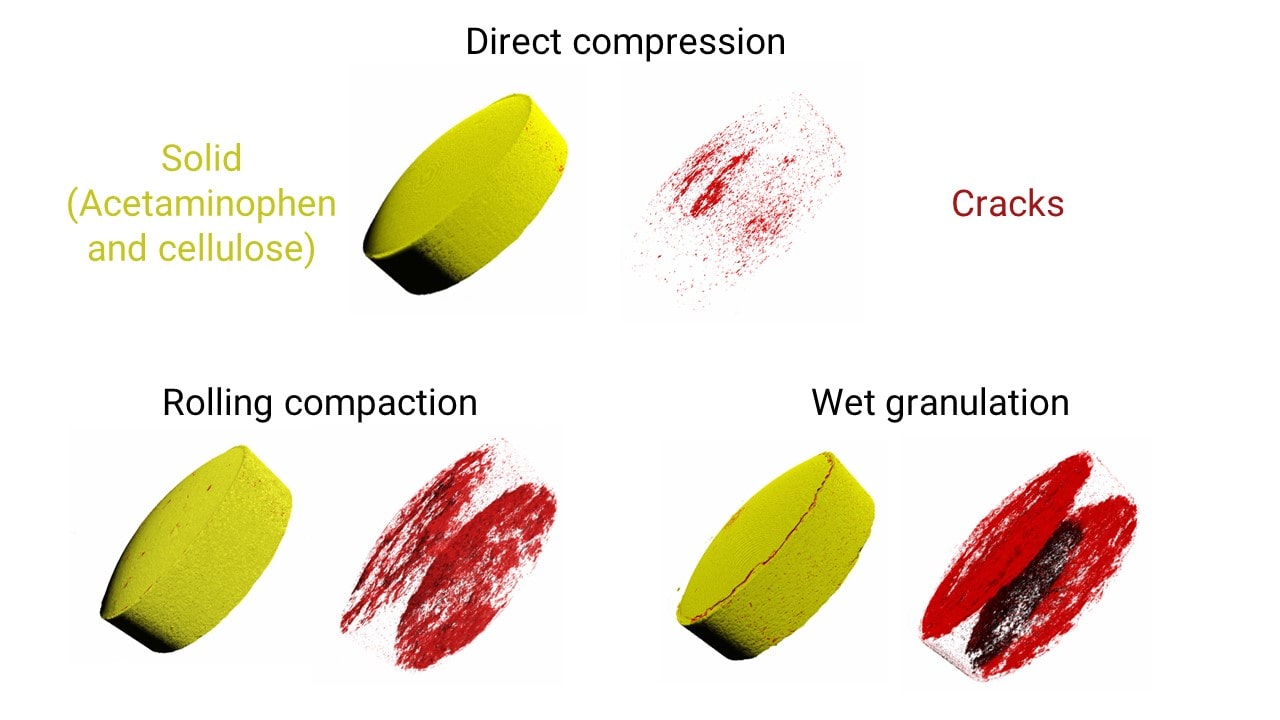
Compression methods comparison
Application Note


