DEGRADATION OF SUSTAINED-RELEASE DOSAGE TABLET
About the sample: Sustained-release dosage tablets
Sustained-release dosage is a mechanism used in tablets and capsules to dissolve a drug slowly and provide a steady release of an API (Active Pharmaceutical Ingredient). The release rate is controlled using various formulations. For example, a combination of a rapid resolving matrix and an internal hydrophobic coating can be used to control when the drug is dissolved and how long it continues to release the API. The structure of the tablet needs to be intact for this type of formulation to function properly. However, when left in a harsh environment, hot and humid for example, some materials in the tablets can degrade and hinder the sustained-release dosage mechanism. X-ray CT (computed tomography) can image the interior of these tables non-destructively and help to study the degradation process.
Analysis procedure
- In this example, a sustained-release dosage tablet was scanned before and after the exposure to heat and humidity using a submicron-resolution CT scanner, nano3DX.
- The CT images were compared to observe which components were affected by the heat and humidity.
1. CT scan
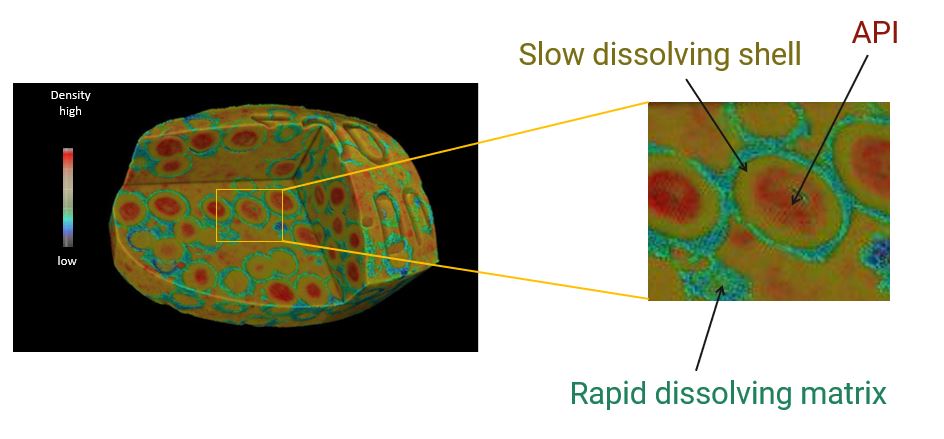
A sustained-release dosage tablet was scanned to produce the 3D grayscale CT image. The intact tablet's CT image is shown in the figure. Three components are colored: the rapid dissolving matrix (green), a slow-dissolving shell (mustard yellow) protecting the API (red).
2. Image comparison
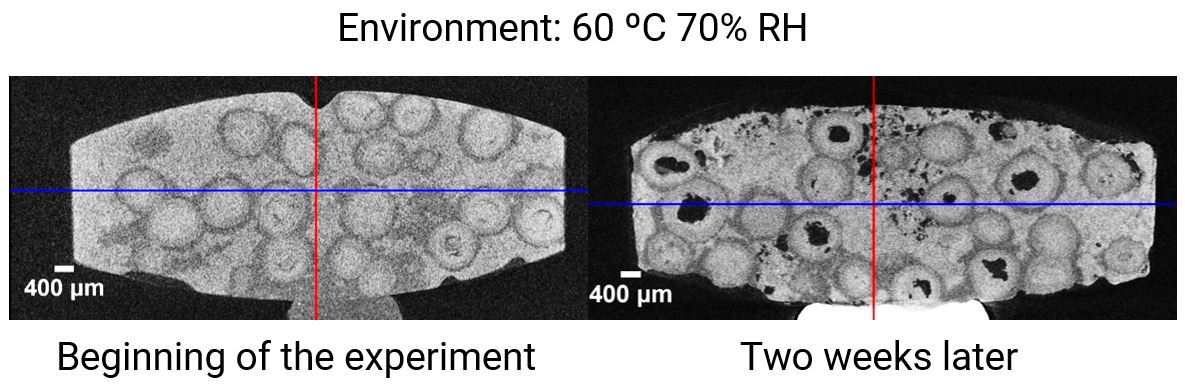
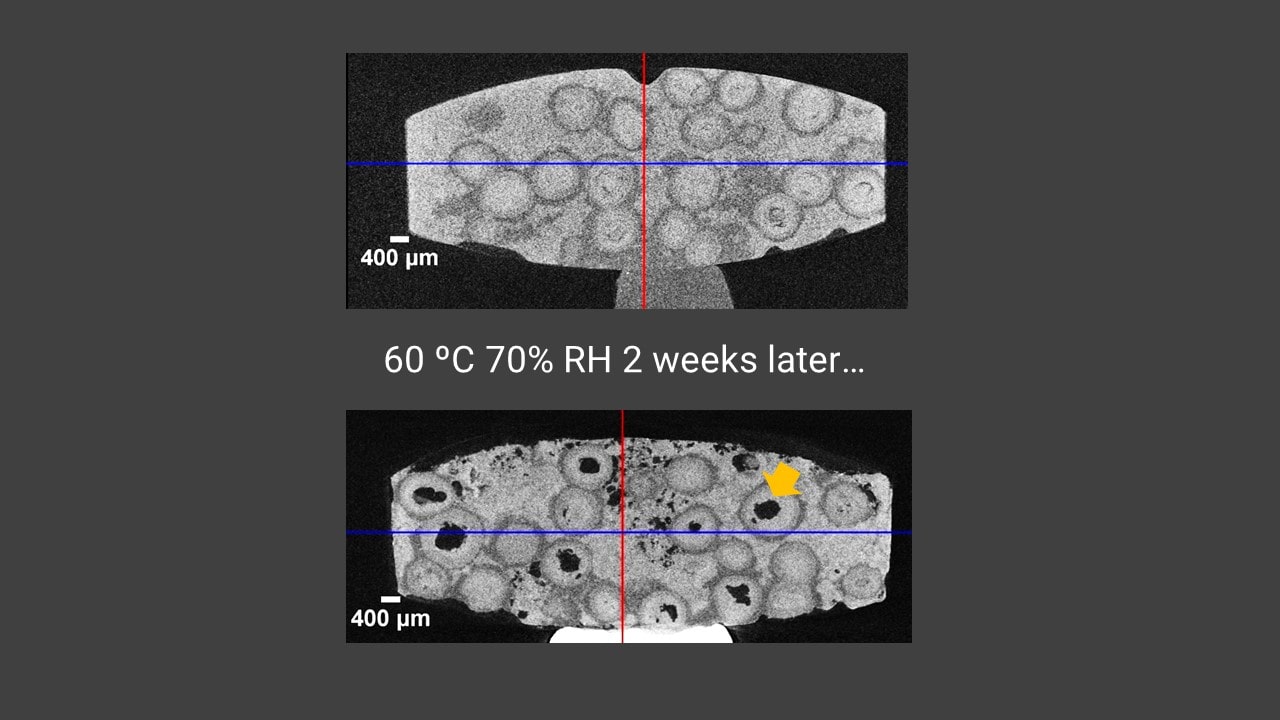
The CT cross-sections of the tablet before and after a two-week exposure to 60 degrees Celsius and 70% relative humidity are shown in the figure. The gray level represents the density of each material, black being the air. At the beginning of the experiment, the tablet was tightly packed and the API cores were well protected by the slow-dissolving shells. After the two-week exposure, many void spaces appeared, indicating that not only the rapid-dissolving matrix but the API cores protected by the slow dissolving shells also dissolved. This result indicates that this particular formulation does not tolerate 60 degrees Celsius and 70% relative humidity exposure for two weeks.
More Pharmaceutical Application Examples
Watch an on-demand webinar about X-ray CT pharmaceutical applications.
Tablet crystallinity analysis
Application Note
Brand name vs generic atorvastatin tablets comparison
Application Note
Microparticle coating analysis
Application Note

Rapid-release pain medication capsule comparison
Application Note
Multivitamin tablet analysis
Application Note
Famotidine tablet comparison
Application Note
Aspirin tablet coating delamination
Application Note
Degradation of sustained-release dosage tablet
Application Note
Compression methods comparison
Application Note


